JBC: Scientists find cellular backup plan for keeping iron levels just right
Iron is essential for cell function, but excess iron can damage cells, so cells have sophisticated molecular mechanisms to sense and adjust iron levels. Disorders of cellular iron metabolism may affect more than a third of the world’s population. In addition disorders like anemia, caused by overall insufficient iron levels, iron deficiency can impair brain function in the young and reduce muscle strength in adults. Iron may be dysregulated at the cellular level in neurological disorders such as Parkinson’s disease, and disordered iron metabolism contributes to congenital conditions such as Friedrich’s ataxia.
Researchers in the nutritional sciences department at the have uncovered a connection in the network of checks and balances underlying cellular iron regulation. was published in the Journal of Biological Chemistry.
When iron levels in mammalian cells are low, iron regulatory proteins, or IRPs, spring into action. IRPs prevent iron that enters cells from being improperly stored, allowing the cell to produce essential iron-containing proteins. When there is excess iron, IRPs are inactive, leading to increased iron storage, lowering potential toxicity and reserving it for when iron availability is reduced. Too much or too little IRP activity can endanger cells.
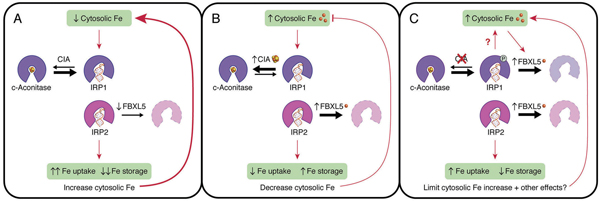 CIA, FBXL5, IRP1 and IRP2 coordinate in control of iron metabolism. Protein degradation and iron-sulfur cluster production can suppress the activity ofiron regulatory proteins, maintaining the correct iron levels in the cell. Courtesy of Caryn Outten, University of South Carolina
CIA, FBXL5, IRP1 and IRP2 coordinate in control of iron metabolism. Protein degradation and iron-sulfur cluster production can suppress the activity ofiron regulatory proteins, maintaining the correct iron levels in the cell. Courtesy of Caryn Outten, University of South Carolina
research group at Wisconsin studies what controls IRP activity. Scientists have long thought the main method by which IRP-1 is inactivated involves essential compounds called iron-sulfur clusters. When there is sufficient iron in the cell, an iron-sulfur cluster is inserted into IRP-1, inactivating it. Thus, the activation or suppression of IRP-1 relates to how much iron is available to produce iron-sulfur clusters.
However, there was some evidence of another method by which IRP-1 could be stopped when it was not needed: namely, that a protein called FBXL5 could add molecular tags to IRP-1 to tell the cell to degrade the protein altogether.
“The idea that IRP1 is also regulated by protein degradation was controversial when it was first discovered by others,” Eisenstein said. “There’s been a belief that IRP-1 was really regulated by this iron-sulfur cluster mechanism, and that the protein degradation mechanism wasn’t so important.”
To test whether this was the case, Eisenstein’s team suppressed the production of iron-sulfur clusters. Even when this production was reduced, IRP-1 activity could still be suppressed. The team confirmed that this was due to the activity of FBXL5. This supported the idea that protein degradation was a backup mechanism that reduced IRP-1 action in cells with high iron.
The results have implications for understanding how iron is sensed, used and regulated in tissues. Different tissues have different levels of oxygen, but the iron-sulfur cluster production system functions best at low oxygen whereas FBXL5 functions best at high oxygen. Therefore, these two systems may trade off taking the lead in controlling IRP-1 in different parts of the body. Because iron-sulfur clusters and FBXL5 play many important roles in cell growth, this balance between these functions could help different types of cells control how they use iron.
“Diseases of iron metabolism caused by diet or by genetic perturbations are major public health issues,” Eisenstein said. “To combat such diseases and develop effective treatments for those afflicted with them, it is essential to understand iron-sensing and iron-regulatory pathways.”
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition weekly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

Elucidating how chemotherapy induces neurotoxicity
Andre Nussenzweig will receive the Bert and Natalie Vallee Award at the 2025 ASBMB Annual Meeting, April 12–15 in Chicago.

Where do we search for the fundamental stuff of life?
Recent books by Thomas Cech and Sara Imari Walker offer two perspectives on where to look for the basic properties that define living things.

UCLA researchers engineer experimental drug for preventing heart failure after heart attacks
This new single-dose therapy blocks a protein that increases inflammation and shows promise in enhancing muscle repair in preclinical models.

The decision to eat may come down to these three neurons
The circuit that connects a hunger-signaling hormone to the jaw to stimulate chewing movements is surprisingly simple, Rockefeller University researchers have found.

Curiosity turned a dietitian into a lipid scientist
Judy Storch will receive the Avanti Award in Lipids at the 2025 ASBMB Annual Meeting, April 12–15 in Chicago.

From receptor research to cancer drug development: The impact of RTKs
Joseph Schlessinger will receive the ASBMB Herbert Tabor Research Award at the 2025 ASBMB Annual meeting, April 12–15 in Chicago.

.jpg?lang=en-US&width=300&height=300&ext=.jpg)