JBC: Zinc homeostasis and autophagy: beyond protein recycling
Zinc is a metal essential for life. In cells, zinc normally is bound to proteins, where it serves as a cofactor, which is essentially a “helper molecule” that aids proper protein function. Absence of zinc can have severe cellular consequences and has been implicated in disease. in the by the lab of Nobel laureate of the Tokyo Institute of Technology revealed that the cellular-degradation system known as autophagy is another consequence of zinc starvation.
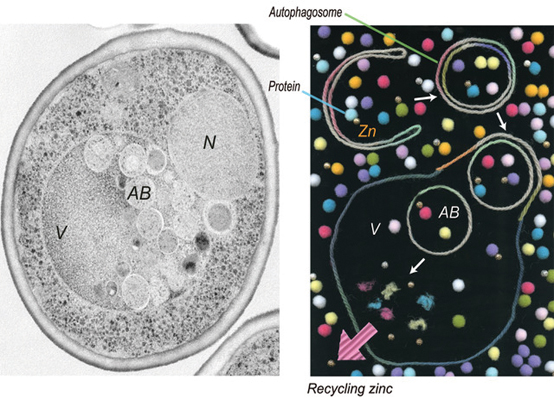 A yeast cell undergoing zinc deprivation-induced autophagy.Image courtesy of the Ohsumi lab
A yeast cell undergoing zinc deprivation-induced autophagy.Image courtesy of the Ohsumi lab
Autophagy is an intracellular degradation process that can function as a recycling system inside the cell. Autophagy occurs at low levels in healthy cells, serving as a quality-control mechanism. Autophagy ramps up when cells are starved for nutrients. The breakdown of intracellular products then provides building blocks for other essential cellular processes.
“Autophagy is often described as a recycling system for proteins,” explains , lead author of the study, “but we recently uncovered that autophagy also functions as a recycling system for metal, such as zinc.”
Previous genetic analysis of zinc-starved cells had revealed that genes related to autophagy are important during zinc starvation. However, the exact role that autophagy plays in zinc starvation remained unknown. “To understand the rules for the induction of autophagy, we tried to dissect how the lack of key nutrients affected growth rate between wild-type and autophagy-deficient cells,” explains Kawamata.
Kawamata’s team grew normal yeast cells and mutant versions deficient in autophagy in a media lacking zinc. The mutant cells’ growth in zinc-free media was impaired significantly compared with normal cells’, suggesting autophagy is important for cellular growth in zinc-depleted conditions.
They also observed that zinc depletion induces autophagy and compared this to what they know about nitrogen starvation, which is thought of as the classical inducer of autophagy. “Compared to autophagy by nitrogen starvation, autophagy by zinc starvation is characterized by a delay in induction,” says Kawamata. “The reason may be that intracellular zinc pools can suppress autophagy for some period of time.” In other words, when starved of zinc, the cells may access their internal stores of the metal to carry on, so autophagy doesn’t kick in right away.
Kawamata then wanted to determine if the zinc starvation-induced autophagy specifically targets the degradation of zinc-containing proteins. She fluorescently tagged a high-abundance protein called Adh1 known to contain zinc, and she tagged two proteins that don’t contain zinc. Then she tracked protein degradation induced by zinc starvation using the fluorescent signals. She found the proteins were degraded on a similar timescale, suggesting autophagy is not selectively going after proteins with zinc.
There’s a lot to learn about the links between external nutrient levels, internal nutrient levels and autophagy. “Our data suggest that induction of autophagy by some forms of nutrient starvation could be closely linked to internal nutrient availability, like the case for zinc starvation,” says Kawamata. This data expands on their recent findings that autophagy plays a role in iron .
Furthermore, the research helps us better understand the role of a crucial nutrient in our cells. Zinc deficiency can lead to irregularities in a variety of organ systems, as zinc-containing proteins are involved with a number of biochemical pathways. “Abnormal zinc homeostatsis causes problems, including disease,” says Kawamata. “We hope our research is useful for understanding diseases linked to this deficiency.”
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition weekly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we鈥檒l send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

From the journals: MCP
Protein analysis of dopaminergic neurons. Predicting immunotherapy responses in lung cancer. ZASP: An efficient proteomics sample prep method. Read about papers on these topics recently published in 麻豆传媒色情片 & Cellular Proteomics.

Unsheathing the role of myelin lipids in Alzheimer鈥檚 disease
Xianlin Han, an ASBMB Breakthroughs speaker, discussed his pioneering work on lipidomics and the role of sulfatide lipids in Alzheimer's disease.

Ten interesting quotes from the JBC archives
Older papers include archaic quirks and long-abandoned biological concepts. Some show flashes of ideas that grew into their own fields, and others show that some things never change.

Lipid biomarkers hold clues to stroke recovery
Scientists at the University of Arizona found that a lipid mediator accumulates with the waves of inflammation associated with stroke and foamy macrophages.

From the JBC archives: Madness, indoles and mercury-based cathartics
A 1907 paper sought to resolve an ongoing question of whether indole, a bacterial molecule in the gut, could cause insanity if overproduced.

From the journals: JBC
Linking modified cysteines to cell migration. Recognizing protein tags for degradation. Disrupting C. difficile toxin production. Read about recent JBC papers on these topics.

