
Listening to ketamine
At 32, Raquel Bennett was looking for a reason to live. She’d struggled with severe depression for more than a decade, trying multiple antidepressants and years of talk therapy. The treatment helped, but not enough to make it seem worth living with a debilitating mental illness, she says. “I was desperate.”
In 2002, following a friend’s suggestion, Bennett received an injection of ketamine, an anesthetic and psychedelic party drug also known as Special K. During her first ketamine trip, Bennett hallucinated that God inserted a giant golden key into her ear, turning on her brain. “It was as if I was living in a dark house and suddenly the lights came on,” she says. “Suddenly everything seemed illuminated.”
The drug lifted Bennett’s depression and dispelled her thoughts of suicide within minutes. The effect lasted for several months, and, she says, the respite saved her life. She was fascinated by the drug’s rapid effects and went on to earn a doctoral degree in psychology, writing her dissertation about ketamine. Today, she works at a clinic in Berkeley, California, that specializes in using ketamine to treat depression. “This medicine works differently and better than any other medication I’ve tried,” she says.

When Bennett experimented with ketamine, the notion of using a psychedelic rave drug for depression was still decidedly fringe.
Since the first clinical trials in the early 2000s, however, dozens of studies have shown that a low dose of ketamine delivered via IV can , including thoughts of suicide, within hours.
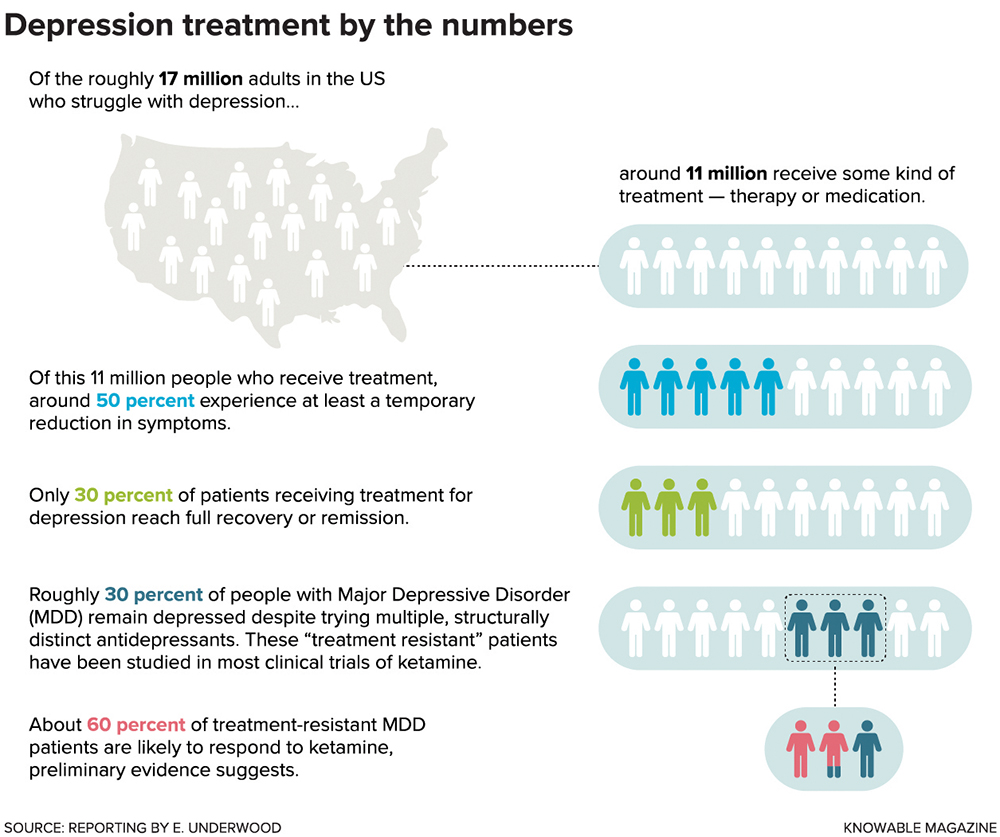
Even a low dose can have intense side effects, such as the sensation of being outside one’s body, vivid hallucinations, confusion and nausea. The antidepressant effects of ketamine typically don’t last more than a week or two. But the drug appears to work where no others have — in the roughly 30 percent of people with major depression who, like Bennett, don’t respond to other treatments. It also works fast, a major advantage for suicidal patients who can’t wait weeks for traditional antidepressants to kick in.
When you prescribe Prozac, you have to convince people that it’s worth taking a medication for several weeks,” says John Krystal, a psychiatrist and neuroscientist at Yale University in New Haven, Connecticut. “With ketamine, patients may feel better that day, or by the next morning.”
The buzz around ketamine can drown out just how little is known about the drug. In the April 2017 JAMA Psychiatry, the American Psychiatric Association published an analysis of the evidence for ketamine treatment noting that there are few published data on the safety of repeated use, although studies of ketamine abusers — who typically use much higher doses — show that the drug can cause memory loss and bladder damage. Most clinical trials of the low dose used for depression have looked at only a single dose, following up on patients for just a week or two, so scientists don’t know if it’s safe to take the drug repeatedly over long periods. But that’s exactly what might be necessary to keep depression at bay.
The analysis also warned about ketamine’s well-established potential for abuse. Used recreationally, large doses of the drug are known to be addictive — there’s some evidence that ketamine can bind to opioid receptors, raising alarms that even low doses could lead to dependence.
Bennett has now been receiving regular ketamine injections for 17 years, with few negative side effects, she says. She doesn’t consider herself addicted to ketamine because she feels no desire to take it between scheduled appointments. But she does feel dependent on the drug, in the same way that a person with high blood pressure takes medication for hypertension, she says.
Still, she acknowledges what most clinicians and researchers contend: There simply aren’t enough data to know what the optimal dose for depression is, who is most likely to benefit from ketamine treatment and what long-term treatment should look like. “There’s a lot that we don’t know about how to use this tool,” Bennett says. “What’s the best dose? What’s the best route of administration? How frequently do you give ketamine treatment? What does maintenance look like? Is it OK to use this in an ongoing way?”
Despite the unknowns, pharmaceutical companies have been racing to bring the first ketamine-based antidepressant to market. In March, the US Food and Drug Administration approved a ketamine-derived nasal spray, esketamine, developed by Janssen Pharmaceuticals, a subsidiary of Johnson & Johnson. Only two of Janssen’s five phase III trials had shown a benefit greater than taking a placebo. Still, in February an independent panel recommended FDA approval. That makes ketamine the first novel depression drug to hit the market in more than 50 years, notes Carlos Zarate Jr, a psychiatrist who studies mood disorder therapies at the National Institute of Mental Health.
Thousands of people are already flocking to private clinics like Bennett’s, which provide intravenous ketamine infusions. Because the drug was approved in the 1970s as an anesthetic, physicians can legally provide the drug as an “off-label” depression treatment. Many ketamine clinics have long waiting lists or are so swamped that they aren’t accepting new patients, and Janssen’s nasal spray could rapidly expand access to treatment.
But some researchers worry that the nasal spray won’t solve many of ketamine’s problems and could create new ones. Although the FDA is requiring that the nasal spray be administered only in a certified doctor’s office or clinic, esketamine is “every bit as habit forming as regular ketamine,” and will be difficult to keep out of the hands of abusers, says Scott Thompson, a neuroscientist at the University of Maryland and a coauthor with Zarate of a 2019 review on in the Annual Review of Pharmacology and Toxicology. A nasal spray can’t deliver as precise a dose as an IV infusion, Thompson notes. “If someone has got a cold, they’re not going to get the same dose.”
In Thompson’s view, esketamine holds few advantages over generic ketamine, which costs less than a dollar per dose, although the IV infusions in private clinics often cost hundreds of dollars per visit. Janssen has indicated that each esketamine treatment will range from $590 to $885, not including the .
Zarate and others are still thrilled to see big pharma investing in ketamine, after decades of stalled efforts to find new psychiatric drugs. “As esketamine hits the market, venture capitalists will come up with better versions and move the field forward,” Zarate says. Several drug companies are now testing other ketamine-like compounds in hopes of developing drugs that have its potent antidepressant potential without its psychedelic and dissociative side effects.
Some researchers are also testing whether ketamine works for conditions beyond depression, such as obsessive-compulsive disorder, as well as in specific subsets of patients, such as severely depressed teenagers. Other scientists are using ketamine to help untangle one of the biggest mysteries in neuroscience: What causes depression?
Seeking answers in neural wiring
Thirty years ago, the prevailing thought was that low levels of certain brain chemicals, such as serotonin, caused depression. Boosting those could remove symptoms.
“I felt that depression needed months or weeks of treatment — that the plastic changes involved in the healing process would require weeks to reset themselves,” says Todd Gould, a neuropharmacologist at the University of Maryland and a coauthor of the recent review paper. But ketamine’s speed of action casts doubt on that idea.
Newer evidence suggests that depression is caused by problems in the neural circuits that regulate mood, Gould notes. Much of the evidence for this faulty-wiring hypothesis comes from rodents. Starting in the 1990s, scientists began to discover intriguing abnormalities in the brains of mice and rats that had been exposed to certain stressors, such as bullying by a big, aggressive male.
Stress and trauma are strong predictors of depression in people, but scientists can’t ask rats or mice if they are depressed.
Instead, they use behavioral tests for classic depression symptoms such as anhedonia, the inability to take joy in pleasurable activities, Thompson says. Depressed animals “give up easily” in experiments that test their willingness to work for rewards like sugar water, or their interest in the intoxicating scent of a potential mate’s urine. “They can’t be bothered to cross the cage,” he says.
Thompson and others have found that there are fewer connections, or synapses, between neurons that communicate reward signals in the brain in depressed animals. Other labs have found shriveled connections in neuronal circuits key to decision-making, attention and memory. Brain imaging studies in people with depression have also revealed abnormal activity in neural circuits that regulate emotion, suggesting that the findings in rodents may also apply to humans.
If faulty neural connections are to blame for depression, the next question is, “How do we get atrophied neural pathways to regrow?” Krystal says.
Circuit training
The answer, many scientists now believe, is the brain’s most abundant neurotransmitter, glutamate.
Glutamate is the workhorse of the brain. It relays fleeting thoughts and feelings, and enables the formation of memories by strengthening synaptic connections. Glutamate is the reason you can still ride a bike years after you learned, even if you never practiced.
Not all glutamate activity is good. Too much can cause the equivalent of an electrical storm in the brain — a seizure — and chronically high levels may lead to dementia. Abnormalities in glutamate receptors — specialized proteins on the surface of brain cells where glutamate can dock and bind — are linked to a wide array of psychiatric diseases, including depression and schizophrenia.
To maintain balance, cells called inhibitory interneurons act like brakes, releasing a neurotransmitter called GABA that quiets brain activity. Most mind-altering drugs work by changing the balance between GABA and glutamate — amphetamines and PCP enhance glutamate signaling, for example, while alcohol inhibits glutamate and boosts GABA.
By the 1990s, scientists had discovered that ketamine triggers a gush of glutamate in the brain’s prefrontal cortex. This region governs attention and plays an important role in emotional regulation. The out-of-body sensations that some people experience when they take ketamine may occur because this rapid release of glutamate “excites the heck out of a whole bunch of neurons” in the prefrontal cortex, says Bita Moghaddam, a neuroscientist at Oregon Health & Science University who discovered the drug’s glutamate-revving effect on rats while studying schizophrenia.
Scientists aren’t sure yet how ketamine forms stronger neural circuits. But the hypothesis goes roughly like this: When ketamine enters the brain, it causes a short-term burst of neuronal activity that triggers a series of biochemical reactions that create stronger, more plentiful synaptic connections between brain cells.
At first, many researchers thought ketamine’s antidepressant effects relied on a structure located on the surface of neurons, called the NMDA receptor. Like a key that fits into different locks, ketamine can bind to several types of NMDA receptor, making neurons release the excitatory glutamate neurotransmitter.
This hypothesis suffered a blow, however, when several drugs designed to bind to the NMDA receptor (as ketamine does) failed in clinical trials for depression.
Esketamine also complicates the story. Ketamine is made up of two molecules that form mirror images of each other, R- and S-ketamine. Esketamine is made up of just the S form and binds roughly four times as effectively as R-ketamine to the NMDA receptor. Despite acting much more powerfully on the NMDA receptor, studies in rodents suggest that S-ketamine is a less potent antidepressant than R-ketamine, although it’s not yet clear whether or not R-ketamine could work better in humans.
Zarate and others now believe ketamine may work through a different receptor that binds glutamate, called AMPA. By pinpointing which receptor ketamine acts on, researchers hope to develop a similar drug with fewer side effects. One hot lead is a compound called hydroxynorketamine (HNK) — a metabolic byproduct of ketamine that does not affect NMDA receptors but still produces rapid antidepressant effects in rodents. The drug appears to lack ketamine’s disorienting side effects, and Zarate and Gould plan to launch the first small clinical trials to establish HNK’s safety in humans this year, likely in around 70 people. “I think we have a very good drug candidate,” Gould says. (Zarate and Gould, among others, have disclosed that they are listed on patents for HNK, so they stand to share in any future royalties received by their employers.)
Plastic synaptic remodelers
To alter how the brain processes mood, scientists believe ketamine must ultimately change synapses. In experiments in rodents, Ron Duman of Yale University has shown that both ketamine and HNK can harness one of the brain’s most important tools for synaptic remodeling: brain-derived neurotrophic factor, or BDNF.
BDNF is a protein intimately involved in shaping synapses during brain development and throughout the lifespan. Healthy brain function depends on having just the right amount of BDNF in the right place at the right time. Many mental illnesses, including depression, are associated with low or abnormal amounts of the protein. For example, samples of brain tissue from people who have died by suicide often contain abnormally low amounts of BDNF.
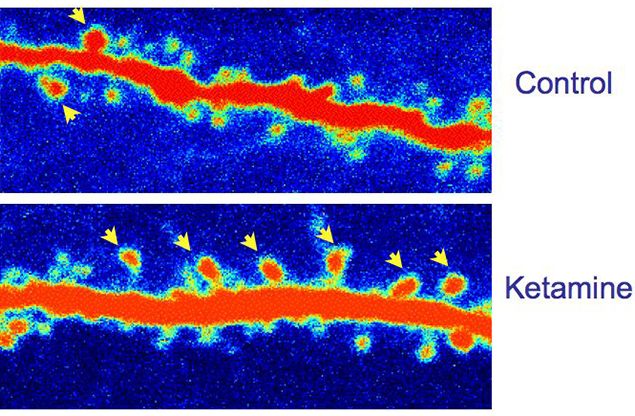
Duman and colleagues have found that both ketamine and HNK cause a sharp uptick in the amount of BDNF that is released from neurons. This increase is required for the drugs’ antidepressant effects, and for the increase in dendritic spines — the stubby protrusions that form synaptic connections with other neurons. Both ketamine and HNK also seem to reduce inflammation, which has been linked repeatedly to the stress-induced loss of synapses.
Ketamine is not the only compound that can induce rapid synaptic plasticity: Other psychedelics, such as ecstasy (MDMA), acid (LSD), and DMT also trigger similar structural changes in neurons and rapid antidepressant effects in rodents, researchers at the University of California at Davis recently found. The effects don’t hinge on getting high, the team . Even very small doses — too low to cause perceptual distortions — can increase synapse density and lift depression.
Traditional antidepressants such as Prozac also increase BDNF levels in the brain, but not nearly as fast as ketamine does, Duman says. That is why most antidepressants take so long to remodel synapses and relieve depression symptoms, he says.
Dissecting depression
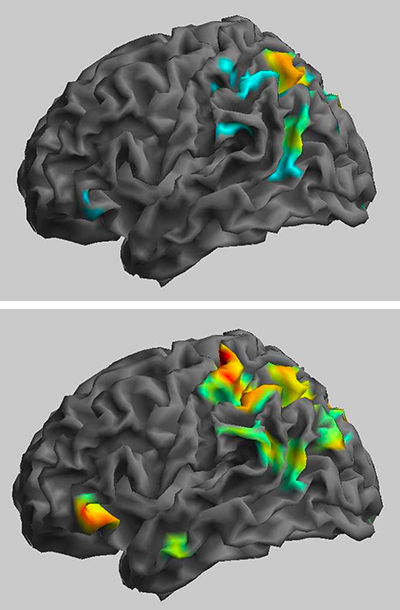
Beyond promising new treatments, Zarate and other researchers see ketamine as a powerful tool for probing depression’s tangled neurobiology. Studies in mice and rats are a good start, but scientists need to study the drug in people to truly understand how ketamine affects the brain. Unlike traditional, slower-acting antidepressants, ketamine lends itself to short-term lab experiments.
Zarate is using neuroimaging tools such as fMRI to study the human brain on ketamine. Past studies have shown that in people with depression, communication among several key brain networks is disrupted. One network, called the default-mode network (DMN), is involved in self-referential thoughts such as ruminating about one’s problems or flaws. This network tends to be hyperactive in people with depression, and less connected to more outwardly attuned brain networks such as the salience network, which helps the brain notice and respond to its surroundings.
In one recent study, Zarate and his colleagues found that after receiving an IV dose of ketamine, people with depression had more normal activity in the default mode network, and that it was better connected to the salience network. At least temporarily, the drug seems to help people get unstuck from patterns of brain activity associated with repetitive, negative thoughts. Zarate does caution that the study results need to be replicated.
The team has also used brain imaging to study how ketamine affects suicidal thoughts. About four hours after an infusion of ketamine, a chunk of the prefrontal cortex that is hyperactive in people with depression had calmed down, researchers found, which correlated with people reporting fewer thoughts of suicide.
Ketamine also seems to tune other brain regions that are key to effective treatment. Last year, scientists published a study in mice showing that ketamine quiets abnormal activity in the lateral habenula, a small nodule wedged deep under the cortex. Some researchers have described the lateral habenula as the brain’s “disappointment center.” The region is responsible for learning from negative experiences, and is hyperactive in people with depression, as if “broadcasting negative feelings and thoughts,” Thompson says.
Such studies remain exploratory. As to why ketamine works — and just as important, why its effects are transient — scientists are still speculating. “I think ketamine is resetting neural circuits in a way that improves the symptoms of depression, but the risk factors — whether genetic, environmental or other risk factors — are still present,” Gould says. “It seems to help reset things temporarily, but the underlying cause is not necessarily resolved.”
Helen Mayberg, a neurologist at Mount Sinai Hospital in New York who specializes in using an experimental procedure called deep brain stimulation to treat depression, suggests that ketamine may be like using a defibrillator on someone experiencing cardiac arrhythmia. “I am not addressing the fact that you have underlying heart disease, but now that your arrhythmia is gone, I can concentrate on other treatments.”
It’s important to put the potential risks of ketamine into perspective, particularly for people contemplating suicide, researchers emphasize. Most people are willing to tolerate severe side effects for other life-saving treatments, such as cancer drugs, Mayberg points out. “If you can interrupt an extreme suicidal plan and ideation, I’ll take that.”
Ketamine in teens?
For Krystal, weighing ketamine’s still largely uncharted risks and potential rewards ultimately comes down to a deeply personal question: “What would we want for ourselves? For our families? Do we want them to have to go through several failed trials over several months, or even a year, before taking a medication that might make their depression better in 24 hours?”
Some of the hardest decisions are likely to involve children and adolescents. Hospitalization for youth suicide attempts and ideation nearly doubled between 2008 and 2015, leaving many clinicians — and parents — desperate for more effective and rapid treatments. Left untreated, depression is “really bad for the brain” and can cause serious, long-term cognitive and developmental problems when it starts young, Zarate says. “The question is, is that going to be better than the long-term side effects of ketamine?”
Scientists don’t yet know. Ketamine has been deemed safe to use as an anesthetic in children, but there aren’t yet sufficient clinical data to show how low, repeated doses of ketamine used for depression could affect the developing brain.
On a more fundamental level, scientists don’t fully understand the neurobiology of adolescent depression, notes psychiatrist Kathryn Cullen of the University of Minnesota. It may involve abnormalities in brain development, such as the way the prefrontal cortex connects to brain regions that process emotion, but “we don’t know if the brain connection abnormalities emerge because of toxic stress induced by depression, or if these abnormalities predispose people to develop depression, or if depression itself reflects abnormal development,” Cullen says. “It’s critical to figure out how to alleviate the biological changes that are associated with [teen] depression so that the brain can get back on a healthy trajectory.”
Two recent clinical trials — one at Yale and another at Minnesota run by Cullen — have found that ketamine can lower symptoms in severely depressed teenagers, but neither study was set up to follow the teenagers long-term, says Cullen. Janssen is currently running a trial of its esketamine nasal spray with 145 youths who are suicidal, but the results of that study have not been published yet.
Cullen thinks ketamine has potential for use in teens, particularly to avoid suicide, but “there are still a lot of unknowns.”
Not just a quick fix
Worldwide, depression afflicts more than 300 million people, making it the leading global cause of disability. When contemplating such overwhelming misery, the vision of a world in which depression can be cured with a single injection or squirt of nasal spray holds obvious appeal.
But — despite the hype — that is not what ketamine offers, Bennett says. Based on her own experience as a patient, and her clinical work, she is troubled by the framing of ketamine as a “rapid” depression treatment if that precludes the slower, more effortful process of psychotherapy. Without psychotherapy, she says, “you’re not giving patients any tools to help themselves, just making them dependent on a molecule that has temporary effects. When the effect wears off, they have to go back for more medicine. This is going to be lucrative for the pharmaceutical company but probably not in the patient’s best interest.”
In Bennett’s clinic, ketamine is administered only alongside talk therapy, which she uses to prepare patients before they take ketamine, and afterward to help them process the experience. “I think this is the only ethical way” to administer a drug that can trigger disorienting psychedelic experiences, she says. “This isn’t a ‘take two and call me in the morning’ situation.”

There’s growing scientific interest in whether ketamine can enhance the effectiveness of therapy by increasing the brain’s ability to remodel circuits through experience, Krystal notes. And in 2017 a small Yale study found that providing cognitive behavioral therapy in tandem with ketamine can extend the drug’s antidepressant effects.
Unlike some researchers and pharmaceutical companies, which consider ketamine’s and esketamine’s hallucinogenic side effects inherently negative, Bennett thinks that for some people the visions can be positive — particularly in the context of therapy. There’s scant scientific evidence to support the idea that such hallucinations are therapeutic, and they can be deeply disturbing for some people. (If people who experience hallucinations do better, it may simply be because they have received a higher dose of ketamine, Krystal points out.)
Still, Bennett thinks researchers and clinicians need to stay open-minded about why ketamine is helping people — and be more attentive to the settings in which ketamine and esketamine are administered. “People consistently report that they experience the presence of God, or their own sacredness,” she says. “When someone comes to my office wanting to kill themselves, ready to die — and then they have a transformational moment where they believe their life is sacred — it’s indescribable how exciting that is as a clinician.”
Depression, fast and slow

In 2001, writer Andrew Solomon published a haunting description of the depression that derailed his early 30s: “If one imagines a soul of iron that weathers with grief and rusts with mild depression, then major depression is the startling collapse of a whole structure,” he wrote.
When Solomon first fell ill, in the 1990s, many clinicians and researchers presumed that the pathological brain changes underlying depression were inherently slow to repair. This mind-set was rooted in the modest but controversial success of a class of slow-acting drugs that includes Prozac.
Developed in the 1950s, the drugs were first inspired by the chance observation that a hypertension drug called reserpine — an extract of the plant Rauwolfia serpentina, or devil pepper — made people intensely depressed. After discovering that reserpine depletes monoamine neurotransmitters in the brain, including serotonin and norepinephrine, scientists hypothesized that low neurotransmitter levels causedepression. They went on to develop monoaminergic antidepressants, drugs designed to increase circulating levels of these chemicals in the brain.
Today, monoaminergic antidepressants include selective serotonin reuptake inhibitors (SSRIs) such as Prozac, Lexapro and Zoloft, as well as the older and less commonly prescribed monoamine oxidase inhibitors (MAOIs) and tricyclic and tetracyclic antidepressants. Scientists have long debated whether the drugs work at all, but the most comprehensive study to date — published in The Lancet in 2018 — suggests that they do lower depression symptoms in about 60 percent of depressed people, albeit only modestly more than taking a placebo.
The benefits start to show up only after several weeks of treatment, however, and roughly a third of people with major depression disorder – called treatment-resistant patients — don’t respond to at least two types of monoaminergic antidepressant.
By the early 2000s, the monoamine hypothesis had unraveled. This was partly due to the antidepressants’ mediocre performance in patients, and partly to experiments which showed that depleting neurotransmitter levels in healthy people does not make people depressed. Scientists now believe that drugs like Prozac do not directly treat depression’s root cause. Instead, they think the drugs work via an indirect mechanism to subtly boost the growth of synapses and the birth of new neurons, and that this somehow relieves symptoms.
Solomon’s bleak metaphor of corrosion was at least partly grounded in science. Many scientists now agree that depression slowly eats away at the neural pathways underlying our sense of worth and well-being, our desire to go to the movies or get out of bed. But research into ketamine holds out new hope that — unlike rusted iron — the depressed brain can be restored, by repairing and strengthening the neural circuits that regulate mood.
—Emily Underwood

Take a deeper dive
Explore related articles from Annual Reviews
This article is republished from , an independent journalistic endeavor from Annual Reviews.
 Emily Underwood is a freelance science writer and contributing correspondent for Science magazine. She is based in Coloma, California. Follow her on Twitter.
Emily Underwood is a freelance science writer and contributing correspondent for Science magazine. She is based in Coloma, California. Follow her on Twitter.Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

Before we’ve lost what we can’t rebuild: Hope for prion disease
Sonia Vallabh and Eric Minikel, a husband-and-wife team racing to cure prion disease, helped develop ION717, an antisense oligonucleotide treatment now in clinical trials. Their mission is personal — and just getting started.

Defeating deletions and duplications
Promising therapeutics for chromosome 15 rare neurodevelopmental disorders, including Angelman syndrome, Dup15q syndrome and Prader–Willi syndrome.

Using 'nature’s mistakes' as a window into Lafora disease
After years of heartbreak, Lafora disease families are fueling glycogen storage research breakthroughs, helping develop therapies that may treat not only Lafora but other related neurological disorders.

Cracking cancer’s code through functional connections
A machine learning–derived protein cofunction network is transforming how scientists understand and uncover relationships between proteins in cancer.

Gaze into the proteomics crystal ball
The 15th International Symposium on Proteomics in the Life Sciences symposium will be held August 17–21 in Cambridge, Massachusetts.

Bacterial enzyme catalyzes body odor compound formation
Researchers identify a skin-resident Staphylococcus hominis dipeptidase involved in creating sulfur-containing secretions. Read more about this recent Journal of Biological Chemistry paper.



