Chasing cancer with dogs
Thunder is a middle-aged mutt with a cheery disposition. He is slim, with a black coat and white patches on his belly and toes. His owner, Jeanneen Terry from Chicago, is unsure of Thunder’s pedigree. “He could be a lab-border collie or lab-pit mix. I don’t know,” she says. “He is a rescue dog.”
Three years ago, Thunder broke his front left leg. A year later, when the leg was still not getting better, his broken leg was amputated, and an X-ray revealed Thunder had osteosarcoma.
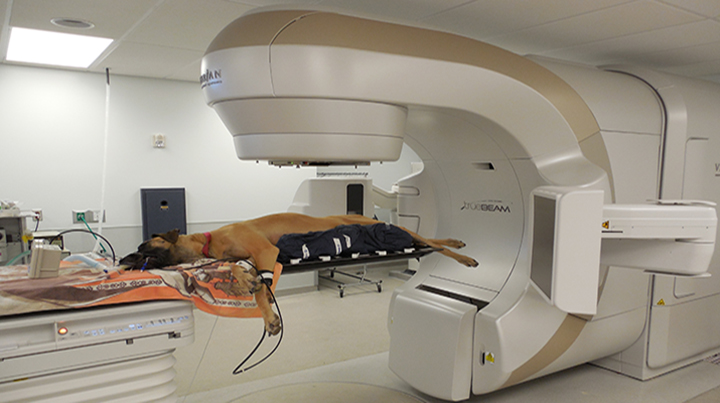 A dog with cancer is positioned for radiotherapy.PHOTO COURTESY OF UNIVERSITY OF CALIFORNIA, DAVIS SCHOOL OF VETERINARY MEDICINE
A dog with cancer is positioned for radiotherapy.PHOTO COURTESY OF UNIVERSITY OF CALIFORNIA, DAVIS SCHOOL OF VETERINARY MEDICINE
 A tumor grew in Thunder’s left front leg.
A tumor grew in Thunder’s left front leg.
Osteosarcoma means “bone tumor” and is the most common and aggressive bone cancer in dogs. It usually spreads rapidly to other organs through the bloodstream, especially to the lungs, and eventually becomes fatal.
Unfortunately, as Terry found out, there aren’t any good treatment options for metastatic canine osteosarcoma. Furthermore, traditional canine cancer treatment is expensive, and she couldn’t afford it. But a friend of hers mentioned a clinical trial program at the College of Veterinary Medicine at the University of Illinois–Urbana Champaign and suggested she look into it. Terry took her friend’s advice and met with the veterinarians who were overseeing the trial program. The doctors assessed Thunder’s eligibility and then invited Terry to enroll him in the program.
The cancer connection in dogs and humans
Cancer is rampant among dogs. According to , executive director of the Duke Cancer Institute, “Cancer kills more than 50 percent of dogs under the age of 10.” Kastan chaired a meeting at the Institute of Medicine in Washington, D.C., this past June on “The role of clinical studies for pets with naturally occurring tumors in translational cancer research.” He says cancer is similarly widespread in humans, occurring at a rate of one in every three women and in half of all men.
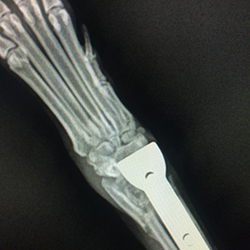 Thunder’s X-ray report revealed osteosarcoma.
Thunder’s X-ray report revealed osteosarcoma.
“It (was) always the interplay between the environment and genetic susceptibility that led to the development of cancer in humans and in dogs,” Kastan said during the meeting’s opening remarks.
Dogs and humans have been living together for thousands of years. They evolved together, eating similar food, breathing the same air, and living in the same homes. , who works on both human and canine genetics at the National Human Genome Research Institute, notes that dogs and humans also have a similar genetic makeup. Although dogs have 38 pairs of chromosomes and humans have 23 pairs, “it’s all the same genes and they are basically in the same order,” Ostrander says.
Not surprisingly, the diagnosis and treatment of cancer in dogs and humans is currently very similar. Dogs frequently get skin, breast, head and neck, brain, testicular, and abdominal cancers, and veterinarians and veterinary oncologists often employ X-rays, blood tests, biopsies and physical exams to detect cancers and surgery, chemotherapy, radiation and immunotherapy to treat them. Traditionally, these protocols have been the result of research done on humans.
Sharing is caring
In 2003, , senior scientist at the National Cancer Institute, spearheaded the comparative oncology program to help researchers assess therapeutic treatments for human cancers by treating pet dogs and cats. Through participation in clinical trials, pets can get free, cutting-edge treatments for naturally occurring cancers. The NCI also established a national infrastructure called the Comparative Oncology Trials Consortium to act as a conduit between the pharmaceutical industry and research institutes. The COTC comprises twenty academic comparative oncology centers in the U.S.
“The clinical trial infrastructure is unique. The whole concept of it is to leverage what we see in naturally occurring dog cancers through the participation of people who seek care,” says , director of the comparative oncology program at the NCI. Academic veterinary centers and participating institutes share data as well as expertise. All of the clinical trial data is added to the system continuously, and it is proving useful for drug companies as they work to move drugs forward in human clinical trials.
For more information, or to enroll a dog, please click .
Looking to dogs for clues
This is where the field of comparative oncology emerges. The National Cancer Institute started the first formal comparative oncology program in the field in 2003 (see “Sharing is caring”) and began undertaking comparative studies of naturally occurring cancers in pet animals and in humans. A few years later, the Comparative Oncology Research Laboratory at UIUC was born.
, a comparative oncology researcher and associate professor at the UIUC’s College of Veterinary Medecine, says, “This discipline is about understanding cancers that are shared between people and dogs and then developing new therapies that will hold promise in people and dogs.”
Proponents say studying the canine version of the disease may help answer questions that have persisted despite studies on mice and humans. One of the main limitations of using mice as a model for cancers is that the disease is not naturally occurring in mice but is induced for the purpose of research. Mice also have a shorter life span, with smaller body size and a different immunological makeup than humans. The tiny size of the mice and their associated tumors make them difficult to analyze repeatedly. In contrast, dogs and humans share similarities in tumor genetics, recurrence, metastasis and therapeutic response. The syngeneic relationships with tumor and microenvironment are also consistent in humans and dogs.
But working with dogs has its disadvantages. Dogs that are enrolled in studies today are only there because their families brought them in. According to Fan, “You can’t order dogs that have cancer.”
When starting experiments, cancer can be induced in many mice at the same time, but with dogs, because the cancer occurs spontaneously, the timing of experiments can’t be controlled. “If you are doing an experiment with dogs with cancer with a new drug, you have to have enrollment of the dogs over a longer course of time,” says Fan. “I can’t control how many dogs are going to come through the front doors of our veterinary teaching hospital.”
Researchers do their best to work around this limitation by enrolling dogs at multiple recruiting sites that are able to participate in the clinical trials.
 Paul Hergenrother and Timothy Fan of UIUC with dog patient Hoover.PHOTO COURTESY OF PAUL HERGENROTHER
Paul Hergenrother and Timothy Fan of UIUC with dog patient Hoover.PHOTO COURTESY OF PAUL HERGENROTHER
Comparing the contrast
Although dogs and humans develop cancers in many of the same ways, there remain interesting differences that could help to answer some oncology questions. For example, there are lots of similarities between dog and human invasive urinary bladder cancers and how they react to common chemotherapy treatments. But an interesting difference between the canine and human versions of the cancer is the presence of a mutation in the BRAF gene. BRAF is an oncogene, and mutation of the gene potentially can cause a normal cell to become cancerous. BRAF is common in the diagnosed dogs, but in humans the mutation is usually not present in urinary bladder cancers but rather in melanomas, colon cancer and thyroid cancer.
The effect of the mutation also seems to be different in dogs and humans. BRAF “seems to be a driver mutation present in dogs but not in humans,” says Heidi Parker, staff scientist at Ostrander’s lab at NHGRI. “But even if you look at mutations in humans, you see the mutations in the same pathway, just not in the same gene.”
Comparative oncology has the potential to stitch together many gaps in our understanding of how the cancer develops and how it can be treated. “The fact that the dog has the identical mutation that’s found in many other kinds of human cancers means that when we are looking at drugs that target this mutation, we could see how they react in dogs with bladder cancer versus how they react in humans, say, with colon cancer,” says Parker.
"PAC"ing a punch
A key characteristic of a cancerous cell is that it can evade apoptosis, or cell death. Avoiding apoptosis makes the cell immortal. Fan and , a chemistry professor at UIUC, figured out a way to deal with the malignant cell’s death-avoiding trick. They discovered a compound called procaspase activating compound, or PAC-1, that induces the activation of procaspase-3 to caspase-3. Caspase-3 is a protease that cleaves critical proteins in the cell and eventually triggers apoptosis. As procaspase-3 is abundant in cancer cells, “we thought activation of it could be very effective and selective for cancer cells,” says Hergenrother.
PAC-1 induced death in cancer cells when used in a clinical trial in dogs with metastatic osteosarcoma, a trial in which Thunder is involved. Thunder was treated with oral PAC-1 along with a chemotherapy agent. After a few weeks, his X-rays “showed that two of the smaller tumors were shrinking and the big ones remained the same, which is a lot better than getting bigger,” says Terry.
Currently, of PAC-1 is being conducted in people with solid tumors or lymphoma at the University of Illinois Cancer Center in Chicago. The goal of the study is to evaluate the maximum tolerable dose of oral PAC-1 in human cancer patients. It’s too early to say what the study results might show.
At the same time, Fan is conducting a clinical trial evaluating the effect of PAC-1 in dogs with brain cancer. “The reason why we are pursuing the evaluation in brain cancer is that PAC-1 gets into the brain, which is something that is relatively unique,” says Fan. Therapies that are currently available for brain cancer are not very effective, so “PAC-1 has the ability to meet an unmet clinical need,” adds Fan.
Main hurdles to overcome
Experts in comparative oncology are enthusiastic about the field’s potential. “It’s an exciting time for us,” says Amy LeBlanc, director of the comparative oncology program at the National Cancer Institute. But she notes there still needs to be more advocacy to enroll pets into clinical trials and to provide better access to the genomic data from canine tumors. LeBlanc says getting more funding is a pressing challenge, as is creating a canine version of The Cancer Genome Atlas that NCI could host. The dog counterpart of the atlas would catalog canine cancer-causing genetic mutations in a comprehensive way for researchers who are using genomics and bioinformatics to understand various forms of the disease in the animals.
For pet owners like Terry, comparative oncology has proven helpful and affordable. Although the osteosarcoma persists and has spread to his lungs, Thunder has enough lung capacity to breathe and doesn’t show too many signs of respiratory distress. He is thin but still enjoying his favorite food and drink – sardines and raw goat’s milk.
Terry says she has no regrets about placing Thunder in a clinical drug trial. “I know that cancer will lead to his ultimate demise, but he will not have gone without a fight and without contributing a little bit to science.”
 Thunder, whose osteosarcoma was treated with PAC-1, after his amputation. PHOTOS OF THUNDER COURTESY OF JEANNEEN TERRY
Thunder, whose osteosarcoma was treated with PAC-1, after his amputation. PHOTOS OF THUNDER COURTESY OF JEANNEEN TERRY
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition weekly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

How antioxidant-enhanced ice cream is changing the game for frozen treats
University of Wisconsin scientists create a sweet treat with more nutrients and better stability using tannins

Helping underrepresented scientists feel seen
Benjamin Garcia will receive the ASBMB Ruth Kirschstein Diversity in Science Award at the ASBMB Annual meeting, April 12–15 in Chicago.

Plants get a GMO glow-up
Lose the prickles. Add bioluminescence. Up the nutrients without the bitter flavor. CRISPR gene editing is opening a new world of genetically modified plants for home growers.

Bananapocalypse – the tricky genetics of a devastating fungus
Fusarium oxysporum can infect over 120 plant species. Whether it destroys Cavendish bananas as it did their predecessor depends on the agricultural industry and consumers.

From the journals: MCP
Young proteins are more likely to be degraded. Full automation speeds up sample preparation. Proteomics sheds light on cancer immune suppression. Read about recent MCP papers on these topics.

Modified pea proteins are shaping the future of meat alternatives
University of Minnesota scientists use enzymes to mimic beef texture in plant-based protein.

