Engineering cells to broadcast their behavior can help scientists study their inner workings
Waves are . Whether it’s the rise and fall of ocean tides or the swinging of a clock’s pendulum, the predictable rhythms of waves create a signal that is easy to track and distinguish from other types of signals.
Electronic devices use radio waves to send and receive data, like your laptop and Wi-Fi router or cellphone and cell tower. Similarly, scientists can use a different type of wave to transmit a different type of data: signals from the invisible processes and dynamics underlying how cells make decisions.
I am a , and my developed a technology that traveling through a human cell to provide a window into the hidden activities that power cells when they’re healthy and harm cells when they go haywire.
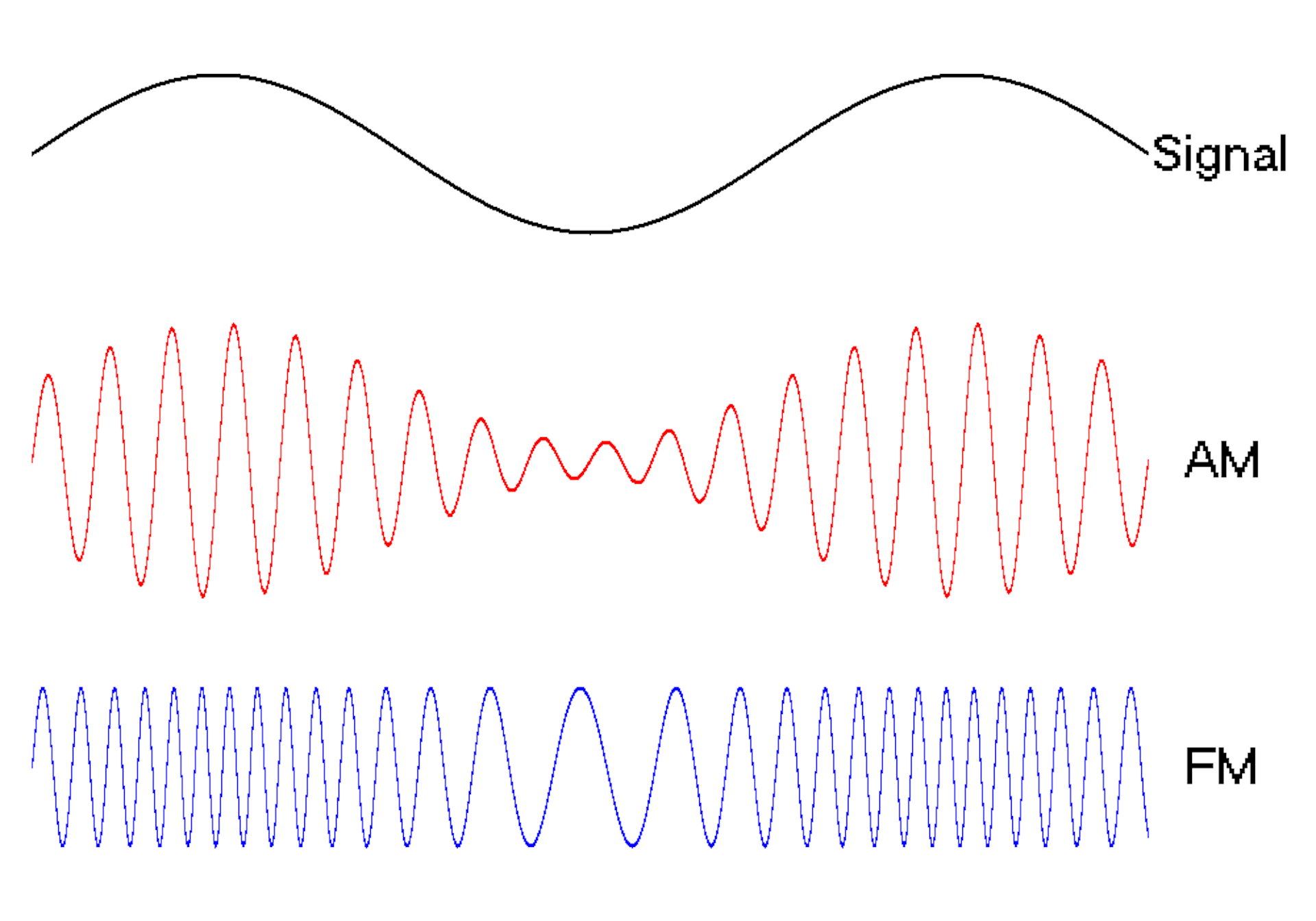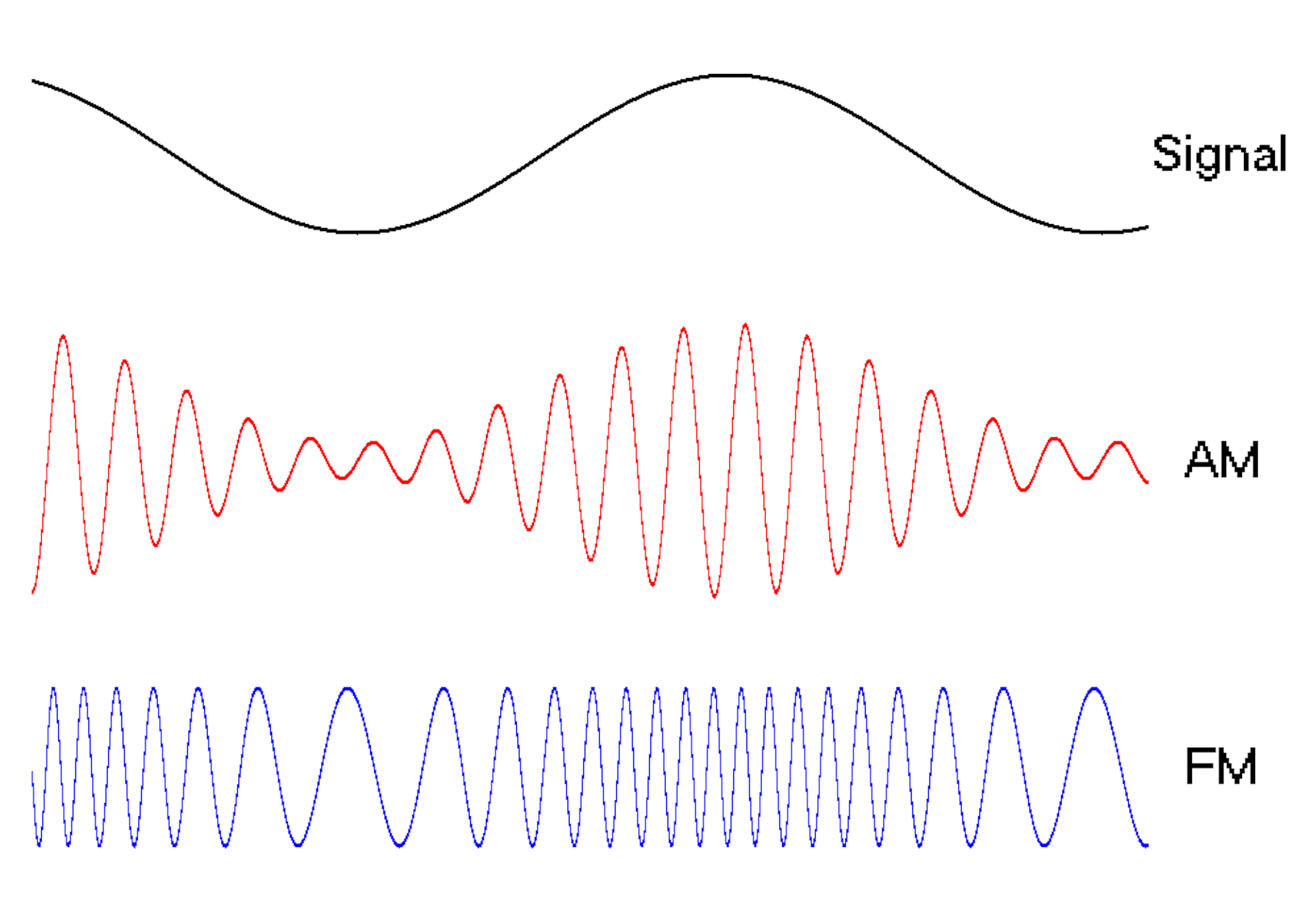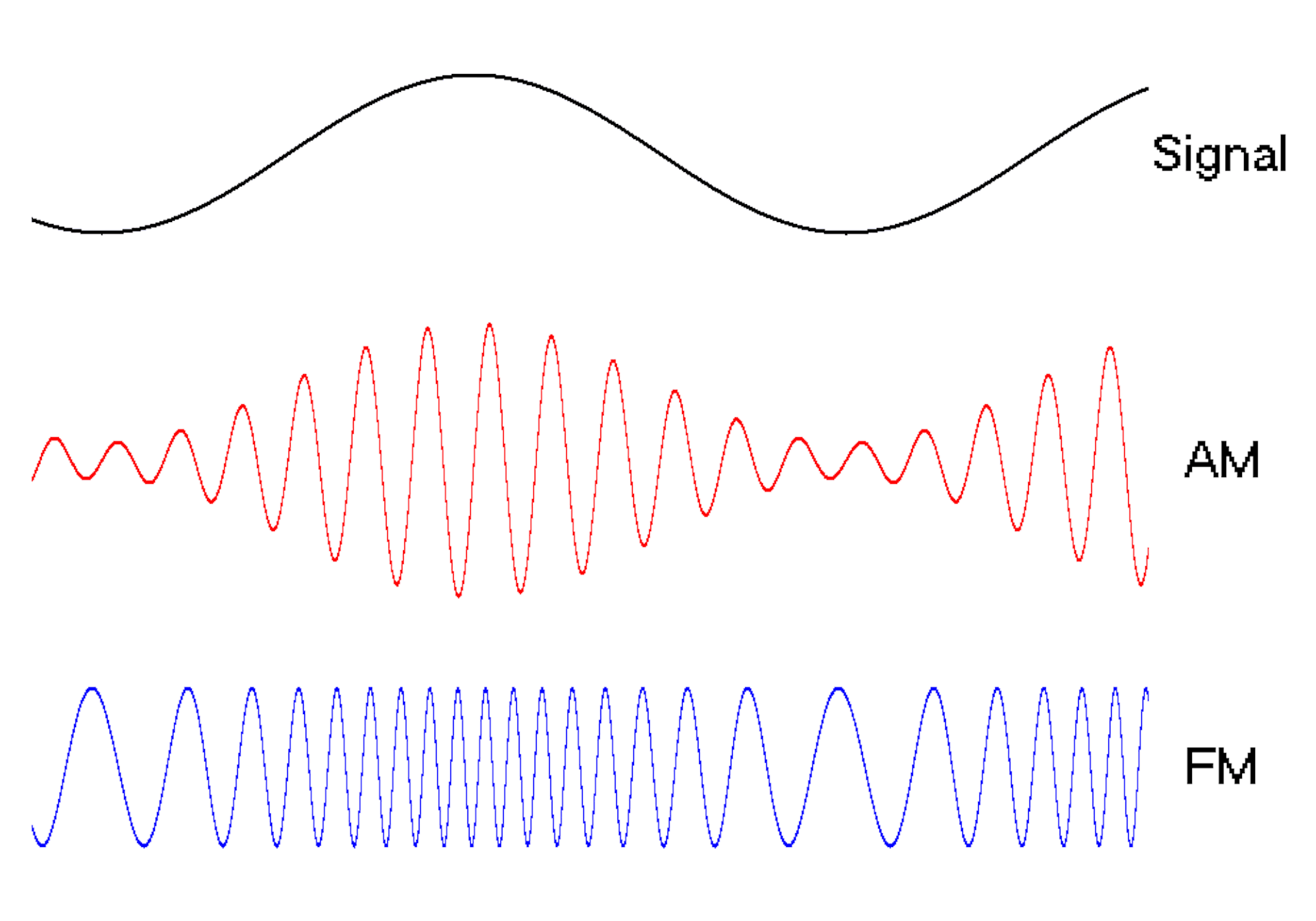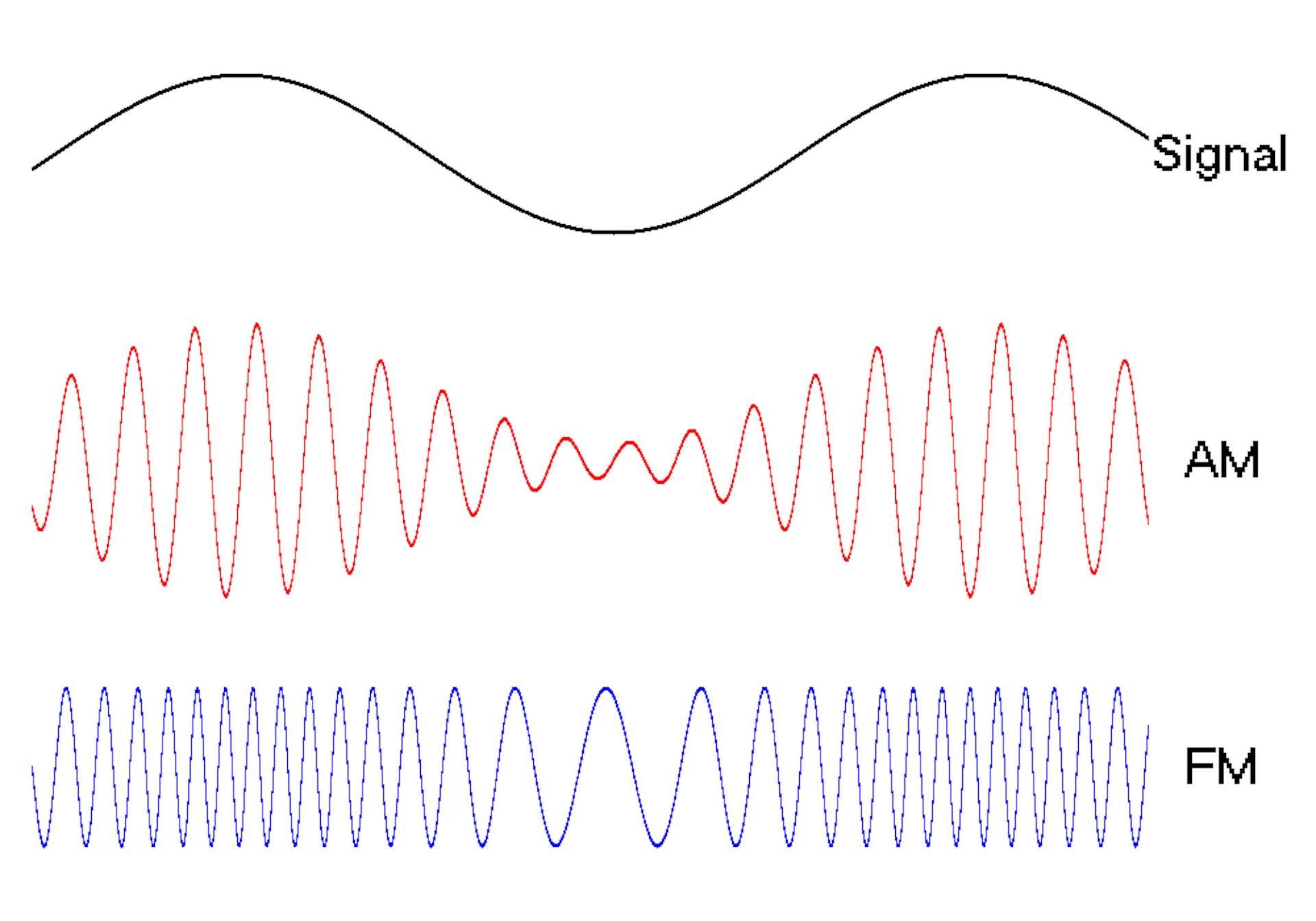
Waves are a powerful engineering tool
The oscillating behavior of waves is one reason they’re powerful patterns in engineering.
For example, controlled and predictable changes to wave oscillations can be used to encode data, such as voice or video information. In the case of is assigned a unique electromagnetic wave that oscillates at its own frequency. These are the numbers you see on the radio dial.
Scientists can extend this strategy to living cells. My team used to turn a cell into a microscopic radio station, broadcasting data about its activity in real time to study its behavior.

Turning cells into radio stations
Studying the inside of cells requires a kind of wave that can specifically connect to and interact with the machinery and components of a cell.
While electronic devices are built from wires and transistors, cells are built from and controlled by a diverse collection of chemical building blocks . Proteins perform an array of functions within the cell, from extracting energy from sugar to deciding whether the cell should grow.
Protein waves are generally rare in nature, but some bacteria naturally generate waves of two proteins called – typically referred to together as MinDE – to help them divide. My team discovered that putting MinDE into human cells causes the proteins to reorganize themselves into a stunning array of .
On their own, MinDE protein waves do not interact with other proteins in human cells. However, we found that MinDE could be to react to the activity of specific human proteins responsible for making decisions about whether to grow, send signals to neighboring cells, move around and divide.
The protein dynamics driving these cellular functions are typically difficult to detect and study in living cells because the activity of proteins is generally invisible to even high-power microscopes. The disruption of these protein patterns cancers and developmental disorders.
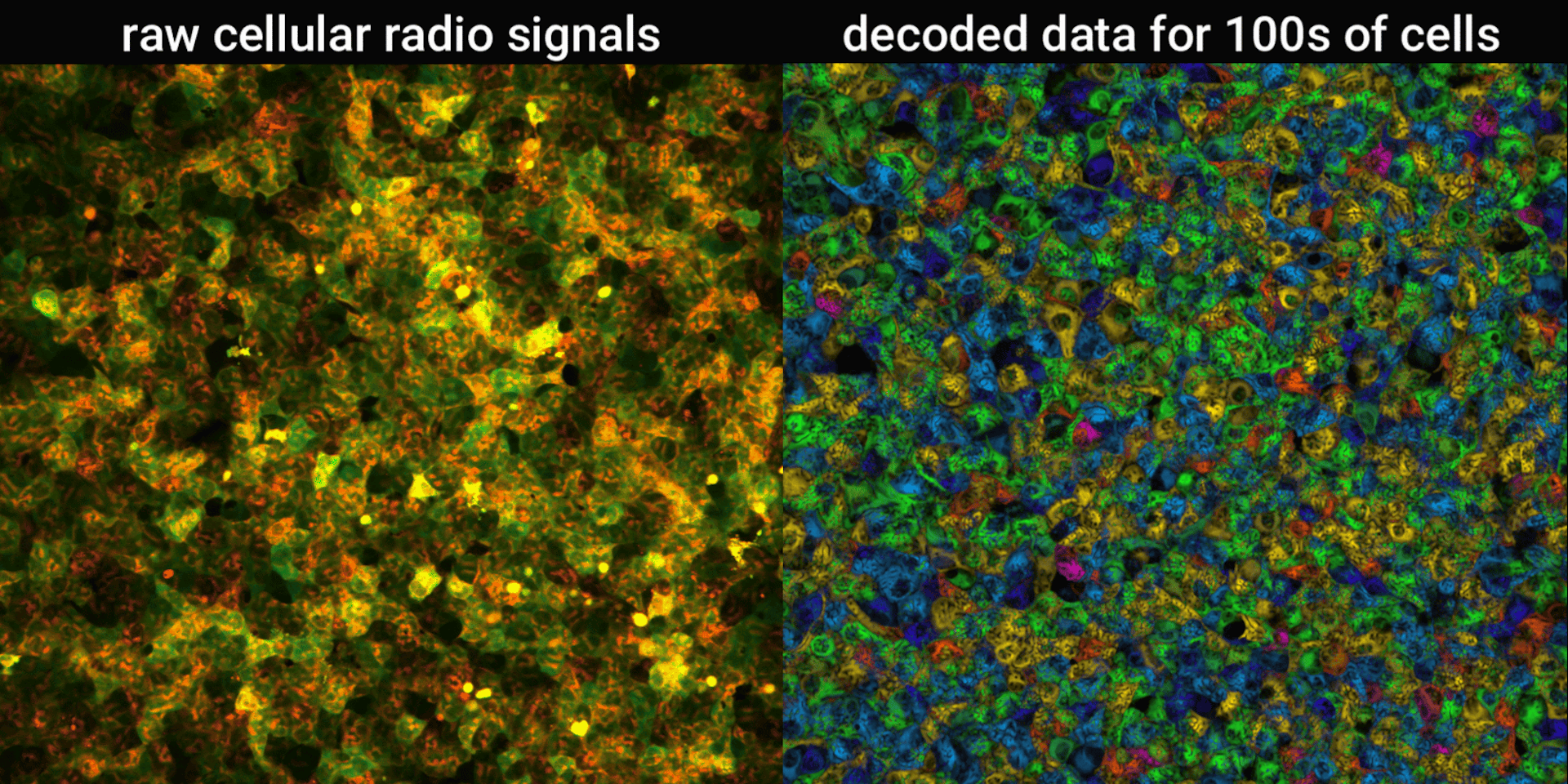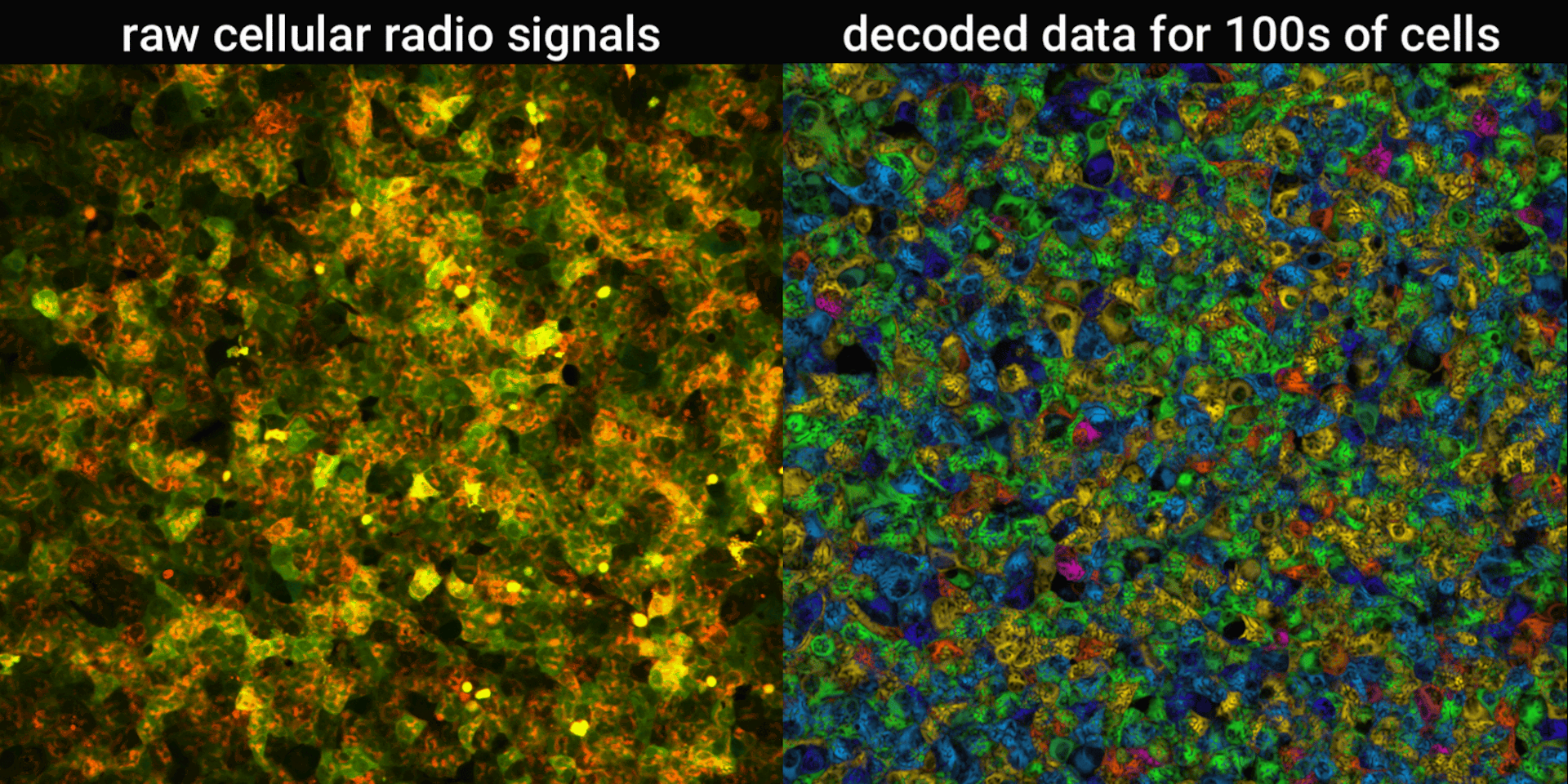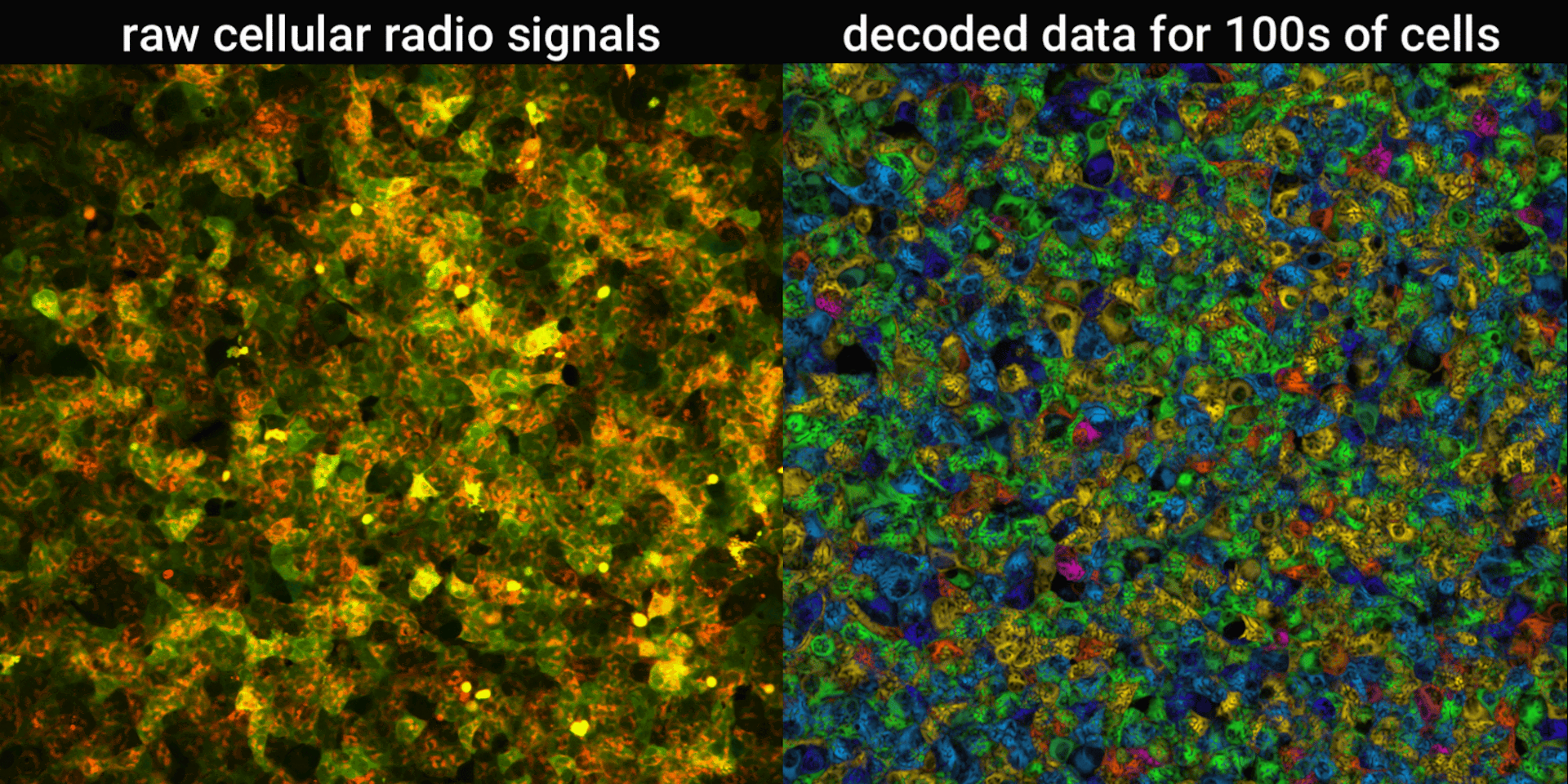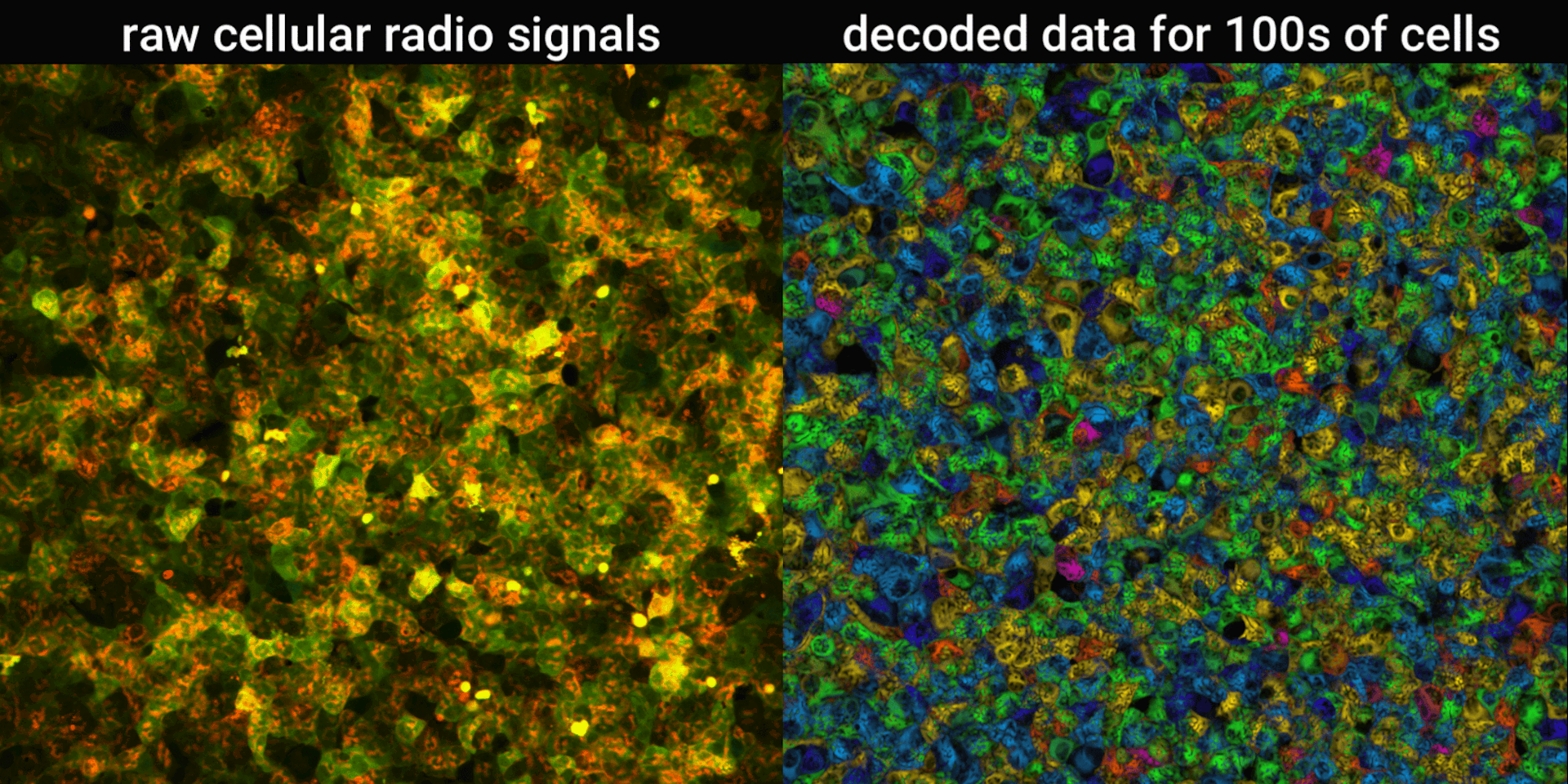
We engineered connections between MinDE protein waves and the activity of proteins responsible for key cellular processes. Now, the activity of these proteins trigger changes in the frequency or amplitude of the protein wave, just like an AM/FM radio. Using microscopes, we can detect and record the unique signals individual cells are broadcasting and then decode them to recover the dynamics of these cellular processes.
We have only begun to scratch the surface of how scientists can use protein waves to study cells. If the history of waves in technology is any indicator, their potential is vast.
This article is republished from under a Creative Commons license. Read the .
![]()
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition monthly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

Mapping the placenta’s hormone network
Study uncovers how the placenta actively metabolizes not only glucocorticoids but also novel androgens and progesterones, reshaping our understanding of pregnancy and its complications.

Biochemists and molecular biologists sweep major 2025 honors
Recent Nobel, MacArthur and Kimberly Prize honorees highlight the power of biochemistry and molecular biology to drive discovery, including immune tolerance, vaccine design and metabolic disease, and to advance medicine and improve human health.

Spider-like proteins spin defenses to control immunity
Researchers from Utrecht University discovered two distinct binding modes of a spider-shaped immune inhibitor found in serum.

A biological camera: How AI is transforming retinal imaging
AI is helping clinicians see a more detailed view into the eye, allowing them to detect diabetic retinopathy earlier and expand access through tele-ophthalmology. These advances could help millions see a clearer future.

AI in the lab: The power of smarter questions
An assistant professor discusses AI's evolution from a buzzword to a trusted research partner. It helps streamline reviews, troubleshoot code, save time and spark ideas, but its success relies on combining AI with expertise and critical thinking.

Training AI to uncover novel antimicrobials
Antibiotic resistance kills millions, but César de la Fuente’s lab is fighting back. By pairing AI with human insight, researchers are uncovering hidden antimicrobial peptides across the tree of life with a 93% success rate against deadly pathogens.

