JBC: A spring-loaded sensor for cholesterol in cells
 Researchers at the University of New South Wales have discovered that a spring-shaped protein structure senses cholesterol in cells.Courtesy of Andrew J. Brown, University of New South Wales Although too much cholesterol is bad for your health, some cholesterol is essential. Most of the cholesterol that the human body needs is manufactured in its own cells in a synthesis process consisting of more than 20 steps. from the University of New South Wales in Sydney, Australia, published in the Journal of Biological Chemistry, explains how an enzyme responsible for one of these steps acts as a kind of thermostat that responds to and adjusts levels of cholesterol in the cell. This insight could lead to new strategies for combating high cholesterol.
Researchers at the University of New South Wales have discovered that a spring-shaped protein structure senses cholesterol in cells.Courtesy of Andrew J. Brown, University of New South Wales Although too much cholesterol is bad for your health, some cholesterol is essential. Most of the cholesterol that the human body needs is manufactured in its own cells in a synthesis process consisting of more than 20 steps. from the University of New South Wales in Sydney, Australia, published in the Journal of Biological Chemistry, explains how an enzyme responsible for one of these steps acts as a kind of thermostat that responds to and adjusts levels of cholesterol in the cell. This insight could lead to new strategies for combating high cholesterol.
Toward the middle of the assembly line of cholesterol production, an enzyme called squalene monooxygenase, or SM, carries out a slow chemical reaction that sets the pace of cholesterol production. In 2011, laboratory at UNSW discovered that when cholesterol in the cell was high, SM was destroyed and less cholesterol was produced. The new research explains how this process of sensing and destruction happens.
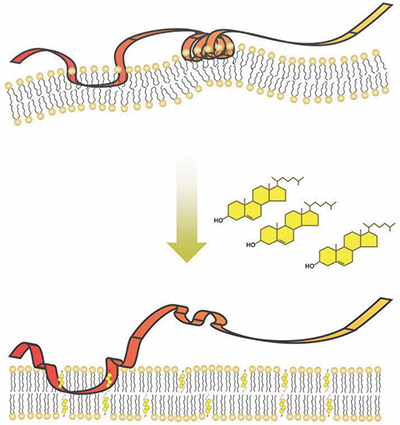
SM is embedded in the membrane of the cell’s endoplasmic reticulum, or ER, which is composed of fatty molecules, including cholesterol. As cholesterol in the cell increases, more and more of it is incorporated into the ER membrane.
SM contains a series of 12 amino acids that serve as a “destruction code” that tells the cell’s garbage disposal machinery to degrade the SM protein. Brown’s team showed that under typical conditions, the destruction code is hidden by being tucked away inside the ER membrane as part of a spring-shaped structure. Using experiments in cell cultures and with isolated proteins and membranes, they also showed that this spring structure could embed only in membranes that contained a low percentage of cholesterol. When the amount of cholesterol making up the membrane increased, the spring popped out, exposing the destruction code.
, a graduate student, led the new study. “When cholesterol levels are low, this destruction code is hidden in the membrane like a spring-loaded trap,” Chua said. “However, too much cholesterol (in the membrane) springs the trap, unmasking the destruction code.”
When this occurs, the cell proceeds to destroy the SM.
The researchers speculate that, because the synthesis step carried out by SM is crucial to determining the amount of cholesterol a cell produces, drugs targeting SM could be used to decrease cholesterol as an alternative to the oft-prescribed statins, which target an enzyme earlier in the cholesterol synthesis assembly line. But they also wonder whether the type of cholesterol-responsive spring they discovered might be used by other proteins involved in cholesterol metabolism to sense and adjust cholesterol levels.
“It’s perhaps stretching the bow a little too far to make a connection from our little cholesterol spring mechanism to metabolic disorders,” Brown said. “But we’ve found a fundamental cholesterol-sensing mechanism, and that’s where this work has advanced the field.”
 Sasha Mushegian is scientific communicator for the Journal of Biological Chemistry. Follow her on .
Sasha Mushegian is scientific communicator for the Journal of Biological Chemistry. Follow her on .Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition weekly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we鈥檒l send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

From the journals: MCP
Protein analysis of dopaminergic neurons. Predicting immunotherapy responses in lung cancer. ZASP: An efficient proteomics sample prep method. Read about papers on these topics recently published in 麻豆传媒色情片 & Cellular Proteomics.

Unsheathing the role of myelin lipids in Alzheimer鈥檚 disease
Xianlin Han, an ASBMB Breakthroughs speaker, discussed his pioneering work on lipidomics and the role of sulfatide lipids in Alzheimer's disease.

Ten interesting quotes from the JBC archives
Older papers include archaic quirks and long-abandoned biological concepts. Some show flashes of ideas that grew into their own fields, and others show that some things never change.

Lipid biomarkers hold clues to stroke recovery
Scientists at the University of Arizona found that a lipid mediator accumulates with the waves of inflammation associated with stroke and foamy macrophages.

From the JBC archives: Madness, indoles and mercury-based cathartics
A 1907 paper sought to resolve an ongoing question of whether indole, a bacterial molecule in the gut, could cause insanity if overproduced.

From the journals: JBC
Linking modified cysteines to cell migration. Recognizing protein tags for degradation. Disrupting C. difficile toxin production. Read about recent JBC papers on these topics.

.jpg?lang=en-US&width=300&height=300&ext=.jpg)