Revamping the Western blot
Ever dreamed of the things you could accomplish if you weren’t stuck baby-sitting a Western blot? Help may be on the way. Some researchers and at least one company are looking to liberate molecular biologists and biochemists from the manually cumbersome and time-consuming process by rethinking the protein immunoblotting technique.
 |
| Robert T. Kennedy’s group developed a microfluidic format that separates and blots proteins onto a membrane. The separated proteins emerge from the arrowhead section of the device. Penny shown for scale. Photo courtesy of Shi Jin of the Kennedy laboratory |
Since its inception in 1979, Western blotting has been a mainstay in molecular biology and biochemistry laboratories and is used as a confirmatory diagnostic for HIV-AIDS. The power of the method lies in its ability to detect a specific protein in a complex mixture. However, the multistep technique takes hours and demands technical skill. It can’t process large numbers of samples at once and requires micrograms of proteins, an amount that can be hard to come by for rare or precious samples. Its performance is inconsistent and gives rise to variable blotting efficiencies, especially with high-molecular-weight proteins.
Instruments are available to speed up some stages of the process, such as automating the incubation steps with antibodies and blocking buffers or improving visualization and quantitation of the final blot. But the fundamentals of “this technique have been around for more than 30 years and have hardly changed. Maybe it’s time people go back and see if there is anything that can be done” to improve it, says at the University of Michigan, Ann Arbor.
Kennedy acknowledges redesigning Western blotting can be controversial, because it’s valid to question whether there is a need to change something that works. “But it’s also true that people spend a lot of time doing Western blots,” says Kennedy.
 |
| ProteinSimple's Simon uses capillary electrophoresis to fully automate the protein immunoblotting process. Photo courtesy of ProteinSimple. |
is a biochemist at Johns Hopkins University who welcomes a redesign. “Traditional Western blotting is tried and trusted, but there are so many limitations to the standard approach. I think the time is right” to introduce some innovations to the process, she states. Rao says, for example, that her laboratory would love to see an increase in the throughput of Western blotting, because often, “we end up designing our experiments around the number of lanes available on a gel, which is not at all ideal!”
Protein research can be shaken up if Western blotting is automated and made faster with the capacity to analyze many samples at once. Today, “there isn’t high-throughput methodology for looking at the protein content and changes in expression and post-translation modifications in large numbers of samples” in an average biology research laboratory, says at the University of California, Berkeley. “There are very few proteins that are currently used in disease diagnostics of any type, and it’s just because looking for protein biomarkers can be really daunting.”
Both Kennedy and Herr envision an automated system where the researcher can just pop in samples and let the instrument do the grunt work. In fact, a new instrument for Western blotting has appeared on the market that matches that description. Furthermore, by increasing the capabilities of Western blotting, those interviewed for the article point out, biologists will be able to formulate more ambitious hypotheses.
 |
| Xingyu Jiang and colleagues created a microfluidic device out of a plastic called PDMS to assay a Western blot with 10 different antibodies at once. Image courtesy of Sha He and Wenying Pan, Jiang Laboratory. |
Capillary electrophoresis meets protein immunoblotting
Capillary electrophoresis is one way investigators are overhauling the Western blot. The method separates molecules by their size-to-charge ratio inside a narrow electrolyte-filled tube and was the workhorse with which the Human Genome Project was completed (1). The advantage of CE is that the sieving matrix for separations can be automatically pumped in and out, because it contains entangled polymers rather than the typical crosslinked polymers of gels, explains Kennedy. He adds, “It’s what made the big difference in the Human Genome Project. It didn’t sound like much, but if you’re talking about running many samples over and over again, that simple automation step made life so much easier.”
CE also requires less sample than gel electrophoresis and has a better resolution of protein size. Kennedy’s laboratory has now swapped the gel electrophoresis step for CE. Proteins travel down a capillary and separate according to size. As the individual proteins emerge at its mouth, they drop onto a blotting membrane moving steadily across the capillary opening. In this way, the researchers drop the time-consuming gel-to-membrane transfer step of conventional immunoblotting and develop the blot as usual. The researchers have shown they can separate classic protein standards like carbonic anhydrase and lysozyme within an hour using only a few nanoliters of sample (2).
In September, the company released the technology in an instrument called . The technology is based on CE and, according to the company, is a “gel-free, blot-free and handsfree solution to the entire Western blotting process.” Mixtures of proteins in nanoliter aliquots are taken into 12 capillaries filled with a sieving matrix and separated by size. The separated proteins are immobilized at the capillary walls in their positions by exposing the capillary to a ultraviolet-light source that activates proprietary chemistry. The separation matrix gets removed, and the reagents for a standard immunoassay flow into the capillary. The primary antibody enters first, followed by a horseradish peroxidase-conjugated secondary antibody, which generates a chemiluminescent readout. Including the time for sample preparation, the totally automated process takes three to five hours, say company representatives.
The instrument allows for quantitative protein measurements. “The biggest challenge with a traditional Western blot performed today is that you separate your proteins in a polymerized acrylamide matrix and transfer it to a solid membrane surface,” says Peter Fung, ProteinSimple’s Simon product manager. “You have no idea how much of your protein that you loaded into that gel is actually transferring to a solid membrane surface. With our technology, we know the proteins that are separated will be captured on the walls of the capillary.”
Trent Basarsky, ProteinSimple’s vice president of corporate development, and Fung both say that their approach gives more reproducible data. No matter “who is in front of the machine, it’s going to give the same answer,” says Basarsky. Although Basarsky declined to reveal the price of the instrument, he and Fung say that the cost of each run in Simon is comparable to that of traditional Western blotting.
Downsizing to microfluidics
The other approach to changing Western blotting is microfluidics, a technology by which small volumes of fluids and molecules move through microscale channels. Kennedy’s group is looking into using microfluidics to further reduce the amount of sample needed for their method and reduce the size of their setup by swapping the centimeter long capillaries for micrometer long microfluidic channels.
and colleagues at the National Center for NanoScience and Technology in Beijing recently incorporated microfluidics for the immunoblotting step. They designed a microfluidic system with channels that allowed 10 different primary antibodies to probe the membrane. Once the incubation step with the primary antibodies was completed, Jiang’s team incubated the whole membrane in a secondary antibody solution. They were able to analyze the expression and molecular weights of 10 proteins, not just a single protein, from a single sample (3). Jiang explains that, because multiple proteins are detected simultaneously, “researchers can save [themselves] the labor of preparing multiple samples.”
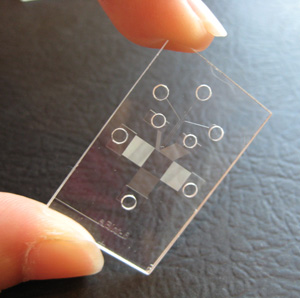 |
| The microfluidic device developed by Amy E. Herr's group does separation, blotting and antibody binding all in one shot. Photo courtesy of Amy Herr. |
Rao says Jiang’s work is an example of how rethinking Western blotting could change the game. She points out biologists now have to strip and reassay blots if they want to test a sample with multiple antibodies, a process fraught with pitfalls. “If you don’t get a signal, you don’t know if the protein is just not there or you lost it” during the stripping process, she says.
Herr’s group hopes to change the entire Western blot procedure with microfluidics. They recently described an automated system made from polyacrylamide gel that automatically does the electrophoretic separation, transfer and blotting all within its confines (4). Herr says the system requires only 0.01 to 0.5 micrograms of protein. She emphasizes that the system is still a prototype, although her team is collaborating with an industrial partner to commercialize it. Herr wouldn’t reveal the company’s identity except to say it was large.
One of the goals of Herr’s team is to determine precisely the absolute abundance of proteins from rare cells. The group is kicking off a project to characterize proteins in mouse hematopoietic stem cells. “Right now, those [cells] are so sparingly available,” says Herr. “There really isn’t any capability for protein or biochemical characterization of proteins, because levels are so small.”
This is the type of application in which , a molecular physiologist at the University of Michigan, Ann Arbor, is very much interested. His laboratory studies glycogen synthase kinase 3’s role in gamete and embryonic development in mammals. Smith explains, “It takes us 300 to 400 oocytes to run one lane on a Western blot. If we do it in triplicate, we’re talking about 1,200 oocytes. Each mouse gives 30 to 40 oocytes.” That’s a lot of dead mice for a Western blot. For this reason, Smith says he is very excited to see the development of miniaturized platforms that could drastically reduce the amount of protein, cells and mice needed for an experiment. Furthermore, both Herr and Smith explain that if microfluidic protein immunoblotting systems let researchers quantify the levels of proteins with different post-translational modifications from just a few cells, and perhaps even single cells, that capability could open new avenues of investigation.
So far, aside from ProteinSimple’s Simon, these methods aren’t commercially available. But the researchers say once they have taken their laboratory prototypes through the development process to become commercial products, they will give biologists the gift of time.
REFERENCES
- Zubritsky, E. How analytical chemists saved the Human Genome Project…or at least gave it a helping hand. (2002) Anal. Chem. 74, 23A – 26A.
- Anderson, G. J., Cipolla, C. M., and Kennedy, R. T. Western blotting using capillary electrophoresis. (2011) Anal. Chem. 83, 1350 – 1355.
- Pan, W., Chen, W., and Jiang, X. Microfluidic Western blot. (2010) Anal. Chem. 82, 3974 – 3976.
- He, M. and Herr, A. E. Polyacrylamide gel photopatterning enables automated protein immunoblotting in a two-dimensional microdevice. (2010) J. Am. Chem. Soc. 132, 2512 – 2513.
Enjoy reading ASBMB Today?
Become a member to receive the print edition four times a year and the digital edition weekly.
Learn moreGet the latest from ASBMB Today
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles

Elucidating how chemotherapy induces neurotoxicity
Andre Nussenzweig will receive the Bert and Natalie Vallee Award at the 2025 ASBMB Annual Meeting, April 12–15 in Chicago.

Where do we search for the fundamental stuff of life?
Recent books by Thomas Cech and Sara Imari Walker offer two perspectives on where to look for the basic properties that define living things.

UCLA researchers engineer experimental drug for preventing heart failure after heart attacks
This new single-dose therapy blocks a protein that increases inflammation and shows promise in enhancing muscle repair in preclinical models.

The decision to eat may come down to these three neurons
The circuit that connects a hunger-signaling hormone to the jaw to stimulate chewing movements is surprisingly simple, Rockefeller University researchers have found.

Curiosity turned a dietitian into a lipid scientist
Judy Storch will receive the Avanti Award in Lipids at the 2025 ASBMB Annual Meeting, April 12–15 in Chicago.

From receptor research to cancer drug development: The impact of RTKs
Joseph Schlessinger will receive the ASBMB Herbert Tabor Research Award at the 2025 ASBMB Annual meeting, April 12–15 in Chicago.

